Low Brain Folate in Autism, Seizures, and Neurological Disorders: Why It’s Overlooked and How to Treat It
Folate is critical for brain development, neurotransmitter production, and neuroprotection. However, many individuals—particularly those with autism spectrum disorder (ASD), epilepsy, schizophrenia, and Parkinson’s disease—experience low folate levels in the brain despite normal blood folate levels. This can be due to folate receptor autoimmunity (FRA), which blocks folate transport across the blood-brain barrier.
Standard folate blood tests do not detect this problem, leading to missed diagnoses and untreated neurological symptoms. While the Walsh Protocol typically avoids folate in undermethylators, this approach does not apply to individuals with low brain folate due to FRA autoimmunity. In such cases, high-dose folinic acid (Leucovorin) therapy is necessary for optimal brain function, particularly in autism, seizures, developmental delay, and movement disorders.
To ensure clarity, the focus of this discussion is primarily on autism and its association with folate receptor autoantibodies, while acknowledging that other neurological disorders, such as epilepsy and Parkinson’s disease, may have related metabolic issues but are not necessarily linked directly to folate autoimmunity.
Why Blood Tests Miss Brain Folate Deficiency
Standard Folate Testing (Systemic Folate, Not Brain-Specific)
- Serum Folate / Plasma Folate / RBC Folate – Measures total folate in blood but not in cerebrospinal fluid (CSF).
- Homocysteine Levels – Elevated levels suggest folate or B12 deficiency but do not indicate brain folate status.
- Macrocytosis (High MCV on CBC Panel) – Can indicate folate deficiency but is not always present in cerebral folate deficiency (CFD).
Brain-Specific Folate Testing (More Accurate but Not Practical)
CSF 5-MTHF Test (Cerebrospinal Fluid Testing)
- Normal CSF Folate Levels: >40 nmol/L
- Mild Deficiency: 30-40 nmol/L
- Severe Deficiency (CFD Diagnosis): <30 nmol/L
- Practical Issue: Requires a lumbar puncture, which is invasive and rarely done.
Autoimmune Testing for FRA Autoantibodies (Best Indicator Without CSF Testing)
Folate Receptor Alpha Autoantibody Test (Serum)
- Detects blocking and binding autoantibodies that prevent folate transport into the brain.
- Found in 60-75% of autistic individuals.
- Available through FRAT.
Conditions Associated with Low Brain Folate
Conditions Linked to Folate Receptor Autoantibodies (FRA Autoimmunity)
- Autism Spectrum Disorder (ASD) – Cognitive impairment, speech delay, seizures, motor dysfunction.
- Cerebral Folate Deficiency (CFD) – Global developmental delay, hypotonia, ataxia, seizures.
- Schizophrenia – Dopamine-serotonin imbalance, cognitive dysfunction.
Conditions Associated with Low Brain Folate (Not Directly Linked to FRA Autoimmunity)
- Parkinson’s Disease – Impaired methylation, elevated homocysteine, oxidative stress, neurodegeneration.
- Epilepsy – Possible folate metabolism disruptions affecting seizure activity.
- Alzheimer’s Disease – Increased oxidative stress, impaired folate metabolism, and mitochondrial dysfunction.
- Depression & Mood Disorders – Impaired methylation leading to neurotransmitter imbalances.
Symptoms Suggesting Low Brain Folate
- Low IQ, speech delay, motor dysfunction, seizures, neurodevelopmental disorders.
- Symptoms unresponsive to typical methylation or neurotransmitter-based therapies.
How to Treat Low Brain Folate in Autism & Neurological Disorders
1. High-Dose Folinic Acid (Leucovorin) Therapy
- Dosage: 1-2 mg/kg/day, up to 50 mg/day (split into two doses).
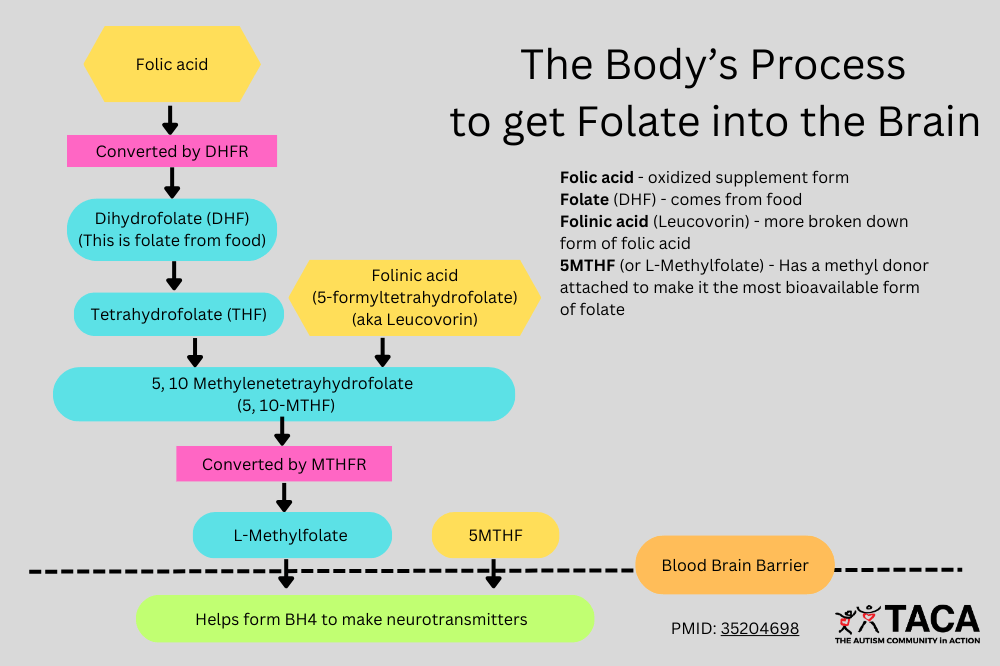
- Why Folinic Acid Instead of 5-MTHF?
- Bypasses FRA autoantibody blockade by using an alternate transport mechanism (Reduced Folate Carrier - RFC).
- Proven in RCTs to improve speech, cognition, and behavior in autistic children (Frye et al., 2018).
2. Improving Gut Health to Reduce Autoimmune Activity
Gut health plays a crucial role in regulating immune responses, reducing autoantibody formation, and improving nutrient absorption, including folate transport.
Diet Modifications to Support Gut Integrity
- Eliminate common inflammatory foods: gluten, dairy, soy, and processed foods.
- Increase fiber intake from whole plant foods, including fruits, vegetables, and resistant starches.
- Consume fermented foods like kimchi, sauerkraut, and kefir to enhance microbiome diversity.
Managing Dysbiosis
- Probiotics with Lactobacillus reuteri, Bifidobacterium longum, and Saccharomyces boulardii help restore gut balance.
- Prebiotic fibers, such as inulin and FOS, support beneficial bacterial growth.
- Herbal antimicrobials like berberine and oregano oil can address overgrowth of harmful gut bacteria.
Addressing Factors that Cause or Restore Leaky Gut
- Reduce environmental toxins such as glyphosate and heavy metals that contribute to gut permeability.
- Support gut lining repair with L-glutamine, collagen, and zinc carnosine.
- Use anti-inflammatory peptides to repair intestinal barrier integrity.
Peptides for Gut Repair & Immune Modulation
- BPC-157 – Repairs gut lining, reduces inflammation, and supports neurotransmitter balance.
- Thymosin Alpha-1 (Tα1) – Lowers autoantibody production, improves immune function.
3. Neurological Protection & Seizure Control
- Cerebrolysin – Neuroprotective, reduces seizures, supports brain repair.
- Vasoactive Intestinal Peptide (VIP) – Improves blood-brain barrier integrity, reduces neuroinflammation.
- Omega-3s (DHA/EPA), Glutathione (NAC), Zinc, and Vitamin D – Support methylation and brain function.
Balancing the Walsh Protocol with Folinic Acid Therapy in Autism
The Walsh Protocol typically avoids folate supplementation in individuals with autism because most autistic individuals are undermethylators, a condition characterized by low serotonin activity and high SERT (serotonin transporter) expression, leading to excessive serotonin clearance. Folate, particularly methylfolate, can worsen this by further increasing SERT and dopamine transporter (DERT) activity, potentially leading to increased anxiety, OCD-like symptoms, emotional instability, and cognitive rigidity.
However, folate receptor autoantibody (FRA) autoimmunity and cerebral folate deficiency (CFD) present a unique and serious exception. In individuals with low brain folate, the neurological consequences—such as seizures, motor dysfunction, cognitive impairment, and speech delays—can be more debilitating than the potential downside of increased serotonin clearance. In such cases, the benefits of folinic acid therapy outweigh the risks, as restoring folate to the brain is essential for neuroprotection, neurotransmitter synthesis, and myelination.
Folinic acid (Leucovorin) is the preferred form of folate for addressing low brain folate in autism because it bypasses folate receptor blockade and provides a usable form of folate without requiring conversion by DHFR. While all forms of folate, including folinic acid and methylfolate, contribute to methylation and may influence SERT and serotonin reuptake, folinic acid converts to 5-MTHF more gradually than methylfolate. This slower conversion reduces the likelihood of abrupt changes in serotonin transporter expression, possibly making it better tolerated in individuals with autism who are sensitive to methylation shifts.
To mitigate any potential negative effects on methylation, additional interventions can support the methyl cycle while using folinic acid therapy:
- Improving SAH (S-adenosylhomocysteine) levels by reducing homocysteine through methyl donors like betaine (TMG), vitamin B6 (P5P), and methylcobalamin (B12).
- Raising systemic alkalinity to optimize methylation enzyme activity through alkaline mineral intake (potassium bicarbonate, magnesium, and calcium citrate) and dietary changes.
- Supplementing with creatine, SAMe, and methionine to support methylation demands without excessive folate metabolism.
- Using co-factors for methylation pathways such as vitamin B12, magnesium and biotin, to ensure balanced neurotransmitter metabolism and homocysteine clearance.
By combining folinic acid therapy with targeted methylation support, the risks of serotonin depletion and exacerbated undermethylation symptoms can be minimized, allowing individuals with autism to benefit from brain folate restoration while maintaining neurotransmitter balance.
Conclusion: Recognizing and Addressing Low Brain Folate in Autism and Neurological Disorders
Low brain folate due to folate receptor autoantibodies (FRA) is a frequent but often overlooked condition, especially in autism, seizures, and other neurological disorders. Standard folate blood tests fail to detect this deficiency, leading to misdiagnosis and untreated cognitive, motor, and behavioral impairments. While the Walsh Protocol typically avoids folate in undermethylators due to its potential to increase SERT/DERT activity and reduce serotonin levels, cases of cerebral folate deficiency (CFD) require an exception. The long-term consequences of untreated low brain folate—developmental regression, speech impairment, seizures, and neuroinflammation—can be more severe than the risks associated with folate therapy.
High-dose folinic acid (Leucovorin) therapy provides a proven treatment strategy for restoring brain folate levels while minimizing disruption to methylation balance. However, caution should be taken in individuals with autism who are undermethylators by supporting methylation pathways concurrently, including raising systemic alkalinity, supplementing with SAMe, creatine, methionine, B12, TMG, and co-factors like magnesium and biotin.
Beyond folate and methylation support therapy, addressing gut health, dysbiosis, and leaky gut is critical in reducing autoimmune activity and optimizing folate metabolism. Eliminating dietary triggers, restoring the microbiome, and repairing the gut lining with targeted nutrients and peptides like BPC-157 and Thymosin Alpha-1 can help modulate immune function and reduce FRA autoantibody production.
By identifying and treating low brain folate early, significant improvements in cognition, speech, motor function, and neurological health can be achieved. The approach must be individualized, balancing the need for brain folate restoration with methylation support, immune modulation, and gut healing to ensure the best possible outcome.