Chronic Low Grade Acidosis and the Need for Alkalinization to Improve Methylation
Modern lifestyles often create a physiological environment prone to chronic, low-grade acidosis. This subtle imbalance arises from dietary habits, physical activity, and common health conditions that disrupt the body's acid-base balance. Unlike severe acute acidosis, which manifests with critical symptoms, chronic acidosis often goes unnoticed but has long-term implications for overall health, cellular function, and enzyme efficiency.
Alkalinization works as a multi-faceted approach to enhancing the body's natural detoxification and metabolic efficiency. By neutralizing acid-forming toxins, it optimizes kidney filtration, reducing the workload required to excrete hydrogen ions and preserving bicarbonate reserves. This improved filtration not only supports the removal of metabolic byproducts but also aids in clearing heavy metals and other acidifying substances. Alkaline environments are crucial for enzyme activation, particularly those involved in detoxification processes, such as liver phase II conjugation pathways, which neutralize toxins for safe excretion. Alkalinization also enhances sweat-based detoxification, making therapies like sauna use more effective by increasing the elimination of acidic compounds and heavy metals through perspiration. Additionally, reducing systemic acidity alleviates the burden on the liver, as less energy is diverted toward acid-base correction, allowing greater efficiency in metabolizing and excreting fat-soluble toxins. By fostering an alkaline internal environment, the body achieves a synergistic improvement in kidney function, enzyme activity, and overall detoxification capacity, resulting in optimized health and resilience against chronic low-grade acidosis and its associated risks.
Causes of Chronic Acidosis
- Dietary Factors:
- High-Protein Diets: Excessive consumption of red meat, eggs, and dairy increases sulfuric acid production from amino acid metabolism.
- Refined Sugars and Processed Foods: These elevate insulin demand and contribute to acidic byproducts during metabolic breakdown.
- Low Mineral Intake: Diets lacking in magnesium, potassium, and calcium from vegetables and fruits fail to neutralize acid loads.
- Lifestyle and Health Conditions:
- Anaerobic Exercise: Intense physical activity in oxygen-deficient states generates lactic acid as a byproduct.
- Sleep Apnea: Intermittent hypoxia during sleep promotes systemic inflammation and acid buildup.
- Chronic Stress: Heightened cortisol levels and reduced nutrient absorption disrupt pH regulation.
- High Altitude: Reduced oxygen availability increases anaerobic metabolism and acid production.
- Environmental and External Contributors:
- Exposure to heavy metals (e.g., lead, cadmium) impairs mitochondrial function, increasing reliance on acid-producing anaerobic pathways.
- Contaminated water, air pollutants, and agricultural residues exacerbate systemic oxidative stress and acid production.
Alkalinization: Dietary and Supplemental Strategies
Diet alone can be sufficient in some cases, especially when rich in alkalizing foods like mineral-dense vegetables (leafy greens, broccoli, kale) and fruits (e.g., avocados, cucumbers). However, given the persistence of lifestyle-induced acidity, sodium bicarbonate and potassium bicarbonate supplementation is often necessary for sustained pH balance. These alkalizing agents:
- Neutralize excess acid in the bloodstream.
- Enhance buffering capacity for lactic acid during exercise.
- Support kidney filtration and prevent the accumulation of harmful metabolites.
Chronic Acidosis: Subclinical and Unmeasurable Concerns
Traditional lab measures, such as sodium, potassium, and general kidney function tests, fail to adequately assess chronic acidity's broader impact on health. Chronic acidity may not immediately disrupt electrolyte balance but subtly influences enzyme activity, mitochondrial efficiency, and detoxification pathways. Over time, these changes contribute to:
- Cancer Risk: Acidic environments promote the proliferation of certain cancer cells.
- Osteoporosis: Chronic acidity leaches calcium from bones to buffer blood pH.
- Cardiovascular Stress: Increased acid load exacerbates oxidative stress and inflammation, contributing to plaque formation.
Why Proactive Alkalinization Matters
Much like drinking adequate water daily supports hydration and detoxification beyond the sensation of thirst, maintaining an optimal pH through alkalinization yields broad, subacute benefits. Regular efforts to neutralize acidity:
- Enhance enzyme-driven metabolic processes.
- Optimize kidney filtration and reduce the workload of acid elimination.
- Improve overall resilience against oxidative stress and inflammatory damage.
By understanding the drivers of chronic acidosis and leveraging dietary and supplemental alkalinization strategies, individuals can promote long-term health and prevent the insidious effects of an acid-prone internal environment.
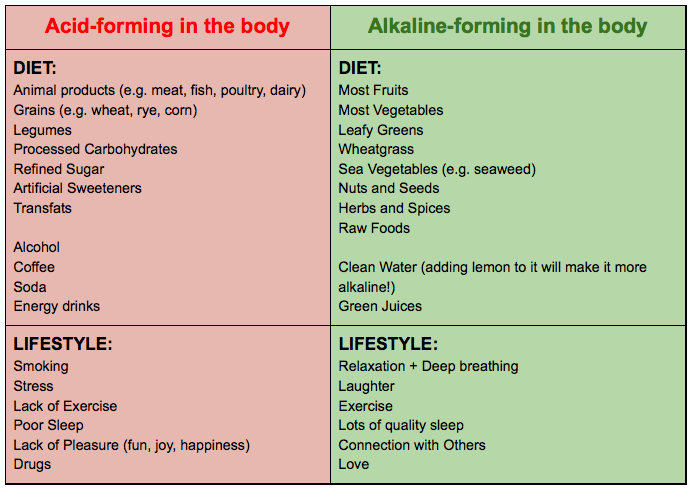
Acid-forming foods, chemicals and other exposures:
Foods
- Animal Products:
- Sulfur-containing amino acids (e.g., methionine, cysteine) in red meat, poultry, eggs, and fish produce sulfuric acid during metabolism.
- Grains and Processed Foods:
- Phosphoric acid (in sodas and processed meats).
- Additives like monosodium glutamate (MSG) and artificial preservatives.
- Sugars and Sweeteners:
- High-fructose corn syrup and refined sugars.
- Dairy Products:
- Lactic acid from fermented products (e.g., yogurt, cheese).
- Alcohol:
- Ethanol metabolism generates acetic acid.
- Fermented Foods:
- Acetic acid (vinegar-based dressings, pickles).
Agriculture
- Pesticides and Herbicides:
- Glyphosate disrupts cellular processes and increases oxidative stress.
- Organophosphates metabolize into acidic byproducts.
- Fertilizers:
- Ammonium sulfate and urea fertilizers release nitrogen compounds that form acids in the soil and groundwater.
- Fungicides:
- Dithiocarbamates and mancozeb introduce acidifying residues into crops.
Body Care
- Preservatives:
- Parabens and formaldehyde releasers disrupt pH balance.
- Synthetic Fragrances:
- Contain phthalates and VOCs that acidify skin and respiratory pathways.
- Antimicrobials:
- Triclosan can alter skin and systemic acid-base balance.
- Hair and Nail Products:
- Acids like glycolic acid (peels) and toluene derivatives in nail polish.
Lawn Care
- Chemical Fertilizers:
- Ammonium nitrate and phosphates acidify soil and runoff.
- Weed Killers:
- Acidic compounds like 2,4-D and glyphosate.
- Pest Control Chemicals:
- Carbamates and pyrethroids produce acidic metabolites.
Atmosphere
- Air Pollution:
- Sulfur dioxide (SO₂) and nitrogen oxides (NOₓ) form acid rain.
- Volatile Organic Compounds (VOCs):
- Released from industrial solvents and vehicle emissions.
- Particulate Matter:
- Fine particles (PM2.5) cause systemic inflammation and acid buildup.
Plastics
- Plasticizers:
- Phthalates disrupt metabolic pathways, leading to acidosis.
- Degradation Products:
- Oxidized compounds from microplastic breakdown.
- Additives:
- Bisphenol A (BPA) interferes with enzyme systems, promoting acidification.
Manufacturing
- Metals and Alloys:
- Heavy metals like lead, cadmium, and mercury accumulate in tissues and impair acid excretion.
- Industrial Solvents:
- Toluene, benzene, and formaldehyde derivatives.
- Acidic Emissions:
- Sulfuric acid and hydrochloric acid fumes from chemical processing plants.
- Paints and Varnishes:
- VOCs and solvents that increase systemic oxidative stress.
Monitoring Subtle pH Changes: A Practical Approach
For tracking small or gradual pH adjustments, urine spot pH and salivary pH offer the most practical and non-invasive options, though their reliability depends on consistent methodology and long-term trend analysis. More sensitive measures, such as venous blood gas or serum bicarbonate, provide better accuracy but are less practical for routine use outside clinical settings.
However, much like hydration, it is often best to assume that alkalinity is beneficial and adopt proactive measures to maintain an optimal internal environment. A practical supplementation strategy includes taking 1 teaspoon of baking soda (sodium bicarbonate) 2–3 times per day and 1–2 potassium bicarbonate tablets twice daily to provide a steady supply of alkaline buffers. These supplements support kidney function, enzyme activity, and overall detoxification, helping the body manage its acid load effectively.
Water and Hydration for Alkalinity
Adequate water intake is essential for supporting kidney filtration and acid excretion. Aim for 64–80 ounces of water per day, depending on activity level and climate. Distilled water, while slightly acidic due to the absence of minerals, lacks harmful acid-forming chemical compounds, such as heavy metals and residues from inadequate filtration systems.
For optimal health, combine distilled water with remineralization supplements or beverages that supply essential minerals. Teas like dandelion and buchu are excellent options for natural remineralization, supporting both hydration and detoxification. Adding ocean mineral drops to distilled water provides trace minerals that further support systemic alkalinity and electrolyte balance.
By combining a remineralized water strategy with supplemental alkalinizing agents, the body achieves enhanced resilience against chronic acidity and its associated health concerns.
Maintaining alkalinity to lower SAH to improve methylation
An alkaline pH environment significantly enhances the body's ability to remove harmful substances like S-adenosylhomocysteine (SAH) and other metabolic byproducts through multiple pathways. Enzymatic detoxification processes, including those involving methylation and sulfuration, are optimized at a slightly alkaline pH, enabling more efficient breakdown and conversion of SAH into less toxic compounds. Alkalinity supports kidney filtration by improving bicarbonate buffering capacity, facilitating the excretion of acidic metabolites and reducing systemic acid load. This also aids the liver by decreasing its detoxification burden, allowing it to focus on processing fat-soluble toxins. Sweat-based detoxification is enhanced in alkaline states, as acidic compounds and heavy metals are more readily excreted through the skin during sweating. Additionally, an alkaline environment reduces oxidative stress, protecting cellular integrity and ensuring that detoxification pathways function without interruption, leading to overall better clearance of metabolic waste and toxins from the body.
